1. Introduction
2. Methods
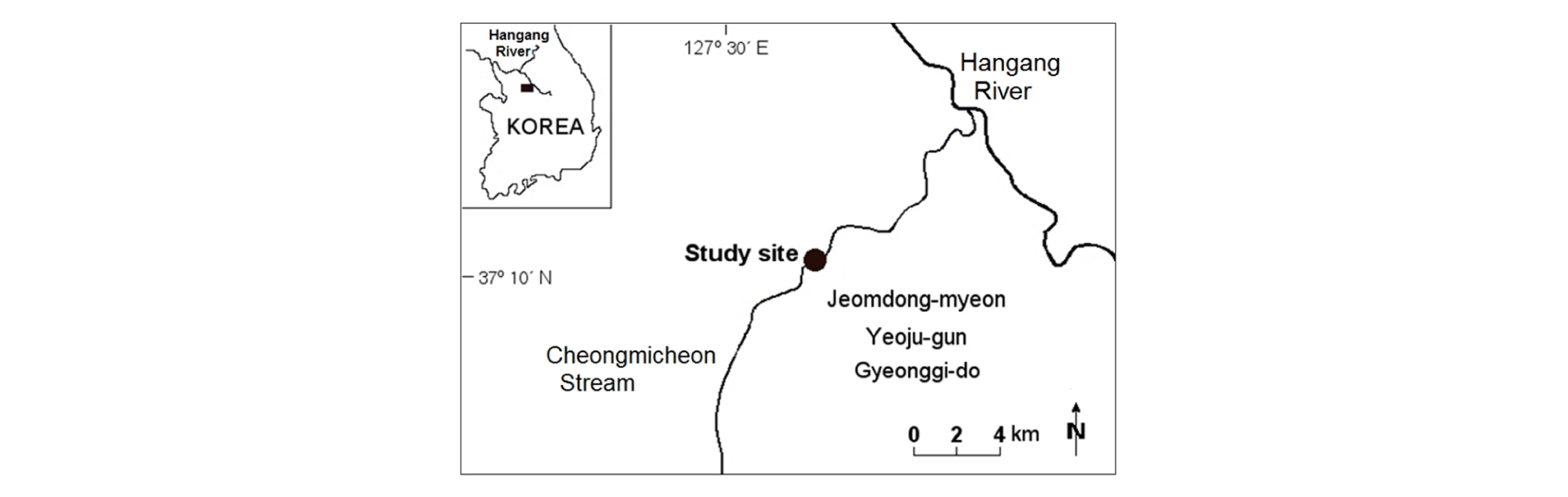
2.1 Study site
2.2 Field survey of soils and vegetation
2.3 Soil seed bank composition
2.4 Data analysis
3. Results
4. Discussion
1. Introduction
Riparian ecosystems, where soils and vegetation are influenced periodically by flooding from an adjacent stream, are unique ecosystems, as consequence of the large fluxes of energy and materials from upstream systems (Gregory et al. 1991, Naiman and Decamps 1997). These ecosystems often exhibit an ecotone between aquatic and upland ecosystems characterized by a sequence of distinct plant communities (Richardson et al. 2007) in distinct zones (Lenssen et al. 1998, Abernethy and Willby 1999, Johansson and Nilsson 2002, Damasceno-Junior et al. 2005, Merritt et al. 2010). This sequence is almost certainly brought about by the over-riding effects of hydro-geomorphic processes (Osterkamp and Hupp 2010), and in particular the magnitudes, frequencies, and durations of stream-flow regimes, all influencing the establishment and survival of plant species in riparian vegetation.
Flooding disturbance regimes, defined by their intensity and frequency, can create different plant communities and landscape mosaics in riverine ecosystems, which vary in time and space across the floodplains (Bornette and Amoros 1996, Shafroth et al. 2002, Garssen et al. 2015). When flood disturbances occur frequently or where chronic damage is brought about by flooding, vegetation succession is interrupted maintaining the riparian vegetation in young successional stages (Tabacchi et al. 1998). Therefore, understanding vegetation regeneration is more important in the floodplains after large flood disturbance in a monsoonal climate (Azami et al. 2004, Cho and Cho 2005).
Central to the temporal changes within floodplain communities is the regeneration niche (sensu Grubb 1977) and regeneration processes, and this includes the soil seed bank. Floodplains soil seed banks are an important reserve of viable seeds, fruits, propagules and other reproductive plant structures (Poiani and Johnson 1989). The soil seed bank is well known to be extremely important for plant community regeneration and conservation in most ecosystems (Bakker et al. 1996, Schoen and Brown 2001). The soil seed bank ecology in riparian zones is perhaps more complex than in other ecosystem types, mainly by interactions between the large number of factors that might impinge on it; including a range of hydrological, geomorphological, and ecological processes all coupled with the direct and indirect impacts of engineering intervention (Goodson et al. 2001). The seed bank has also a significant influence on riparian vegetation structure, which in turn influences the morphology and stability of the riparian habitat (Goodson et al. 2001). Moreover, floods can contribute to increased biodiversity within the streams because they remove above-ground vegetation and erode soil, thus providing new open spaces for the germination or regeneration of plants from the seed bank (Bornette et al. 1998). Even moderate flooding could be an important initiator of change through gap creation and dispersal of plant propagules into and within the riverine ecosystem (Nilsson et al. 1991, Craig and Malanson 1993, Gurnell et al. 2008). If the soil seed bank plays an important role in the regeneration of extant vegetation, there should be considerable similarity in floristic composition between soil seed bank and riparian vegetation in the stream. Nevertheless, little similarity between soil seed bank and extant vegetation has been demonstrated in many studies, but hardly any of these have been carried out under monsoonal climatic conditions (reviewed, Hopfensperger 2007).
In Korea, with a temperate-monsoonal climate, rainfall is concentrated in summer when heavy flooding occurs frequently. The different vegetation types are developed across the floodplains according to hydro- geomorphic gradient in the floodplain (Cho and Cho 2005). Moreover, the riparian vegetation and geomorphology are often disturbed during these large-scale summer floods (Lee et al. 2014). However, after disturbance, the riparian vegetation usually regenerates rapidly over the disturbed floodplains (Lee et al. 2014). It is, therefore, expected that the soil seed bank is fundamental in the regeneration process of riparian vegetation in regularly-disturbed floodplains. The aims of this study were, therefore, to describe the compositional similarities between soil seed bank and above-ground vegetation in a series of six major riverine plant communities and the relationships between species composition of seed bank and environmental factors in the Cheongmicheon Stream, Korea.
2. Methods
2.1 Study site
The study site was located on the Cheongmicheon Stream, a 62 km long tributary of the Hangang River that drains a 594 km2 watershed within the central Korean Peninsula (Longitude 127° 39′ 43′′ E; Latitude 37° 10′ 27′′ N, Fig. 1). The stream originally meandered freely with a very sinuous flow through the plains, but has recently been narrowed and limited by channel straightening and levee construction. The reach studied was 1 km in length with a stream width between levees of 250 m, and a low-water channel width of 35 m. Within the meandering low-flow channel, sandbars are present. The floodplain supports a dense vegetation cover across the stream. The geomorphology, soils, hydrology, and vegetation have been described by Cho and Cho (2005).
2.2 Field survey of soils and vegetation
Soil samples for both physico-chemical and seed bank analysis were taken from six major plant communities present on the floodplain (Cho and Cho 2005). These communities were identifiable via their dominant species, i.e.: Artemisia selengensis, Miscanthus sacchariflorus, Persicaria nodosa, Phalaris arundinacea, Phragmites japonica, and Rorippa palustris. In March 2003, 10 quadrats (1 m × 1 m) were located randomly within each plant community. The species composition in each quadrat was surveyed monthly between March and October 2003. When sampling the plant communities, the average distances from the low water channel and heights above the low water surface were measured to obtain data on their topographical position relative to the stream.
In March 2003, 10 soil cores (diameter 5 cm and depth 5 cm) were sampled randomly from each quadrat within each community as well as an open sand bar. The samples from each quadrat were bulked and mixed thoroughly, and divided into two identical sub-samples per quadrat. One sub-sample per quadrat was selected randomly for seed bank analysis and stored at 4°C for one month until the germination trials were started. Five of the remaining sub-samples from each plant community were air-dried for analysis of soil organic matter and soil texture. Soil organic matter was measured using by loss-on-ignition at 550°C for 4 hours (Heiri et al. 2001). Soil texture, i.e. composition of sand, silt, and clay fractions, was determined using the hydrometer method (Sheldrick and Wang 1993).
2.3 Soil seed bank composition
The soil seed bank was analysed by measuring direct seedling emergence (Lee et al. 2011). The 10 bulked soil samples of each plant community were sprinkled onto the surface of a germination tray (25 cm × 10 cm × 5 cm deep) filled with 3 cm sterile potting compost. The germination trays were maintained in a glasshouse (temperature range 9 - 33°C) from April 2003 for 138 days with daily watering. The positions of the trays were changed randomly every two weeks. All identifiable seedlings were counted and then removed to prevent overcrowding after 19, 49, 75, 107, and 138 days. Seedlings that were difficult to identify were transplanted to separate pots and were allowed to grow until identification was possible. The nomenclature of vascular plant species follows the Korean Plant Names index (Korea Forest Service 2017).
2.4 Data analysis
The relationship in floristic composition between soil seed bank and above-ground vegetation was analysed using Sorensen’s similarity index according to their presence/absence data for the comparison with results of other studies (Hopfensperger 2007). Canonical Correspondence Analysis (CCA) was used to provide an integrated analysis of the relationship between seedling germination and measured environmental variables; the vegetation data matrix was transformed (loge (x + 1)), and removing species that were present in only one sample. An initial analysis indicated that distance from the channel was the only significant environmental variable (p < 0.05), hence the CCA was re-run with this variable as the sole constraining variable. Thereafter, Principal Co-ordinate Analysis (PCO) was used to compare the species composition of the seed bank and the above-ground vegetation (presence/absence data) after calculating the Bray-Curtis dissimilarity index. To help the interpretation standard deviational ellipses of the seed bank and the above- ground vegetation were used (‘ordiellipse’ function; Oksanen et al. 2017). Both PCO and CCA were performed using the ‘vegan’ package (Oksanen et al. 2017) within the R statistical environment (R version 3.4.0, R Core Team 2017).
3. Results
The soils of the study site for the seed bank analysis were composed mainly of alluvial sands, with a sand content ranging from 78 ± 2% for the Phalaris arundinacea community, 86 ± 3% for the Artemisia selengensis community and soils from the other communities were >90% (Table 1). Soil organic matter content was low, ranging from 0.4% (Rorippa palustris) to a maximum for 4.2 ± 0.3% (P. arundinaceae). As the distance and the terrain height increased from the low-flow channel to the levee, communities were located in sequence: (low-flow) Persicaria nodosa, R. palustris, P. arundinacea, Phragmites japonica, Miscanthus sacchariflorus, and A.selengensis (levee) (Table 1). A total 42 of vascular plant species were found in the extant vegetation of the floodplains; eleven of these species (26%) were perennials (Appendix 1).
Table 1. Distance and topographic height from the channel water and soil texture and organic matter content of soils in major vegetation in the floodplains of the Cheongmicheon Stream, South Korea (mean ± SE, n = 5)
Only twenty-one species of vascular plants germinated from the floodplain soil seed banks (Table 2). There was a large divergence in the number of species detected in each community. The communities of R. palustris and P. nodosa communities had relatively few species (2 and 7 respectively) and all others had between 13 (P. arundinacea) and 19 species (A. selengensis). Only one species, Cardamine flexuosa, was found on the open sand bar without vegetation. The most numerous species that germinated were: Alopecurus aequalis in every community and P. nodosa, Capsella bursa-pastoris, C. flexuosa, and Stellaria aquatica in all communities except R. palustris. Almost all the species detected were annuals, with only two perennial, ruderal species, Artemisia princeps and Rumex crispus (Table 2). Of note is the lack of seeds of the dominant species found in the vegetation, for example no seeds of A. selengensis, M. sacchariflorus, P. japonica and P. arundinacea, were detected. The average numbers of viable seeds on the open sand bar were estimated at 51 seeds m-2. Only the R. palustris community had a similar density (102 seeds m-2); all other communities had much greater densities ranging from 969 ± 233 seeds m-2 (mean ± SE) in P. nodosa community through to 10,152 ± 2,515 seeds m-2 in the P. arundinacea community (Table 2)
Table 2. Total numbers of seedlings (seeds m-2) for each species germinated from soils collected in six plant communities in the floodplain of the Cheongmicheon Stream, South Korea. Mean values (±SE, n = 10) are presented
The Canonical Correspondence Analysis (CCA) of the seed bank composition produced eigenvalues of 0.1212 and 0.1309 and explained 26% and 28% of the total inertia on the first two axes, respectively. The species biplot showed (Fig. 2 (a)) that plants preferring a more humid habitat such as A. aequalis, Mazus pumilus, and R. palustris were located at the upper left side and plants preferring a more mesic habitat such as Panicum bisulcatum and Chenopodium ficifolium at the lower right side of the ordination. Only distance from the water channel was selected as a significant environmental factor in the floodplain seed bank (p < 0.05). The seed bank of P. nodosa community and R. palustris community was correlated negatively with the distance from water way (Fig. 2 (b)). The contrast is that the P. nodosa community was distributed at the wide sandy bar, and R. palustris community was restricted on the muddy creeks near the channel. The seed bank of A. selengensis community was located far from the low flow channel at the high terrain.
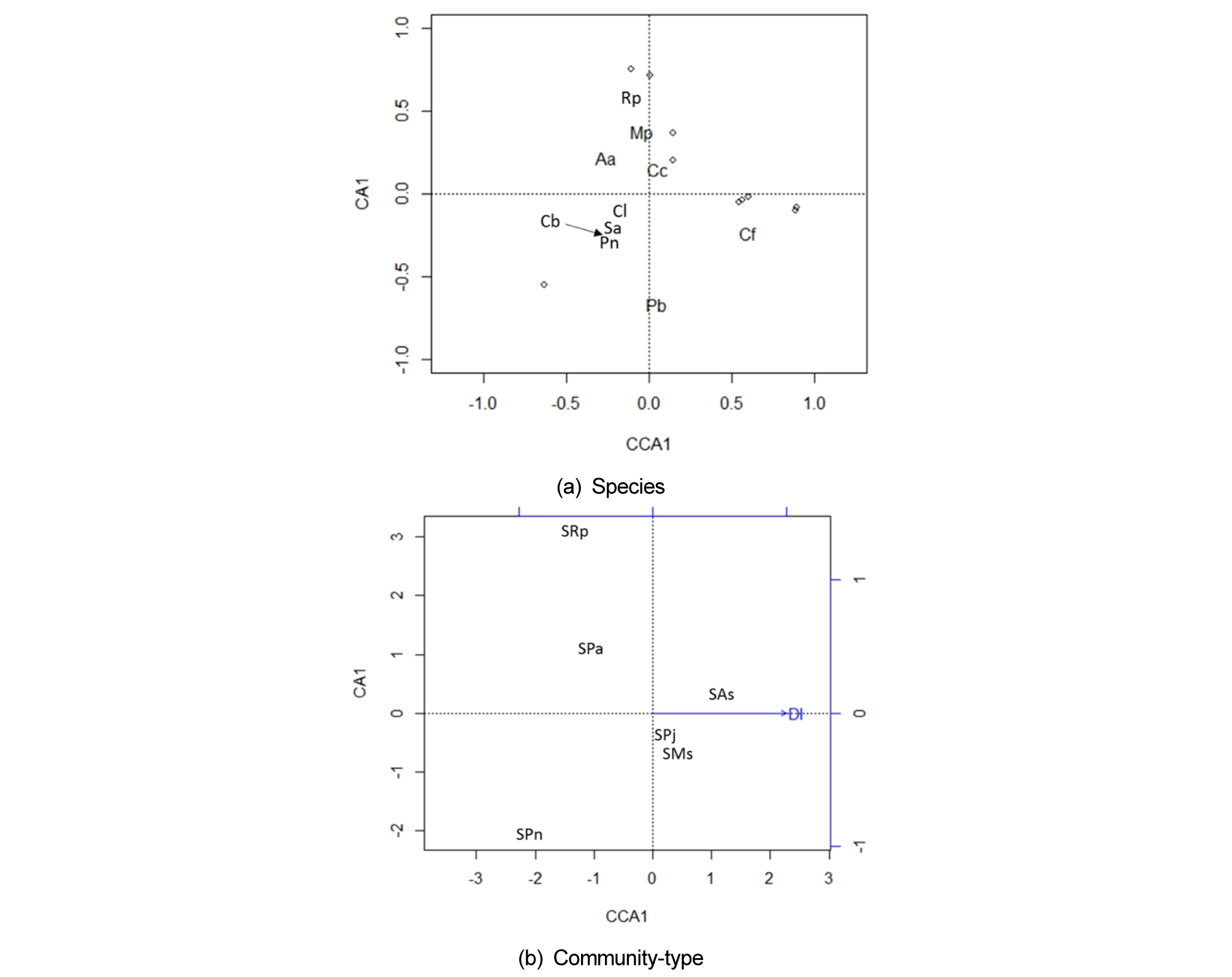
Fig. 2.
Results of the canonical correspondence analysis showing the relation of environmental variable to seed bank composition using seedling numbers in the floodplain of the Cheongmicheon Stream, South Korea: (a) Species biplot, here only species with > 1,000 seedlings are presented as text. (b) Community type in response to distance from the stream channel (DI). Codes: the seed bank is prefixed by S, and the last two letters indicate the plant community. Species: Aa = Alopecurus aequalis, As = Artemisia selengensis, Cb = Capsella bursa-pastoris, Cc = Conyza canadensis, Cf = Chenopodium ficifolium, Cl = Cardamine flexuosa, Mp = Mazus pumilus, Ms = Miscanthus sacchariflorus, Pa = Phalaris arundinacea, Pb = Panicum bisulcatum, Pj = Phragmites japonica, Pn = Persicaria nodosa, Rp = Rorippa palustris, Sa = Stellaria aquatic.
The species present within both the seed bank and above-ground vegetation are presented in Appendix 1. In most communities, more plant species were found in the above-ground vegetation than in the seed bank samples. These differences were variable between communities (Table 3); for example, there was ×4.1 and ×7 more species in the P. nodosa and R. palustris communities respectively, but for the other communities it was much less pronounced (×1 - 1.2). However, the similarity in species composition between the seed bank and above-ground vegetation was relatively low for all communities, ranging from 15 - 40% (Table 3). The Principal Co-ordinate Analysis (PCO) identified distinct differences in the floristic composition between seed bank of soil and above-ground vegetation (Fig. 3). The plotted positions of seed banks were located closer each other than those of above-ground vegetation. The seed bank of R. palustris community, where only two species of seedling were found, was located away from other seed banks.
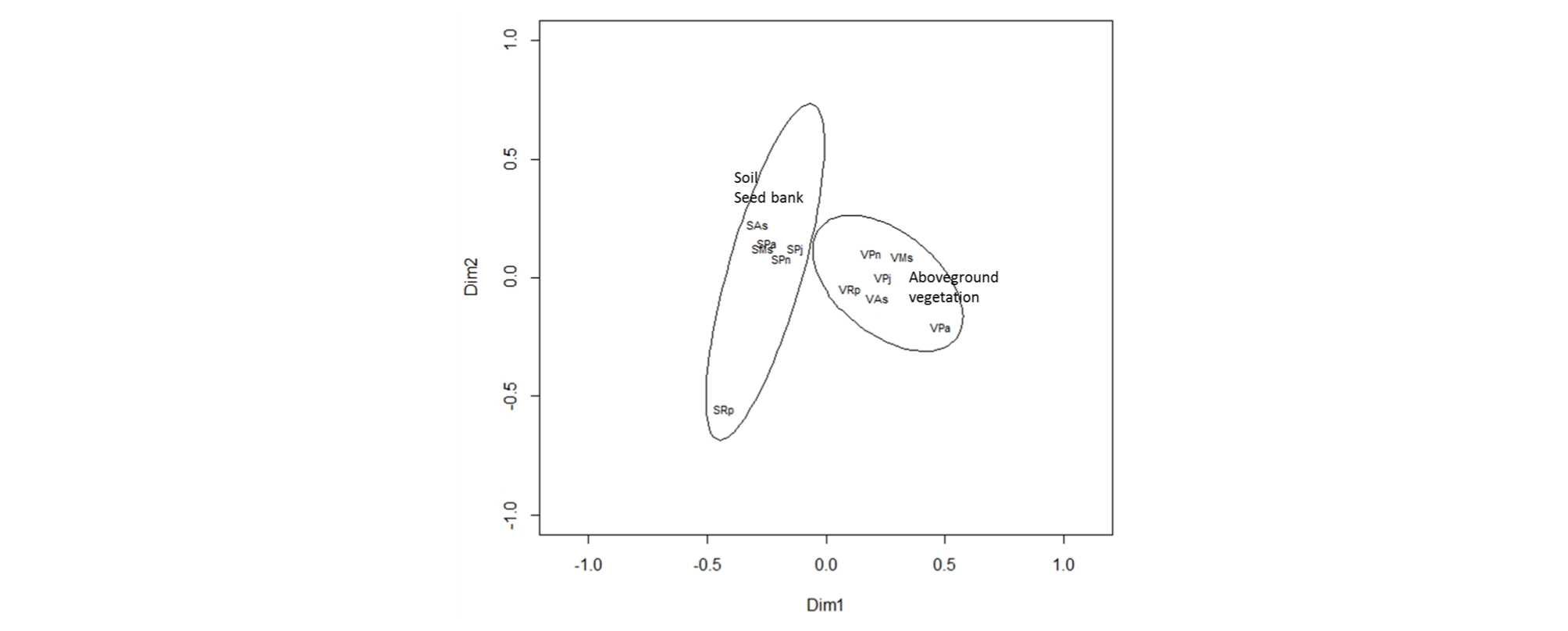
Fig. 3.
Biplot of principal co-ordinate analysis showing the difference between the floristic compositions of soil seed bank and above-ground vegetation in the floodplain of the Cheongmicheon Stream, South Korea. The bivariate- deviational ellipses (95% confidence intervals) indicate the seed bank and above-ground vegetation. Codes: the seed bank and vegetation data are prefixed by S and V respectively, and the last two letters indicate the plant community: As = Artemisia selengensis, Ms = Miscanthus sacchariflorus, Pa = Phalaris arundinacea, Pj =Phragmites japonica, Pn = Persicaria nodosa, Rp = Rorippa palustris.
4. Discussion
This study of seed banks in floodplains highlighted the predominance of pioneer ruderal plants in the species compositions of the soil seed bank and the low similarity of floristic compositions between the soil seed bank and above-ground vegetation in a sand-bed stream within a temperate-monsoonal climate.
There was a successional sequence of plant communities from the open sand bar with no above-ground vegetation through communities dominated by Persicaria nodosa and Rorippa palustris, at the front of sand bar and then Phalaris arundinacea, Phragmites japonica, Miscanthus sacchariflorus, and finally Artemisia selengensis at the high elevation of floodplain near the levee. The dominant species in the three earlier- successional stages were annuals, but the three later-stage dominants species were perennials (Cho and Cho 2005, Lee et al. 2009). The annual communities were located in more frequently-disturbed sites than the later perennial ones. A total of 42 species was found within the above-ground vegetation of these floodplains.
In contrast, only 21 species were detected in soil seed bank as a potential source of plant community regeneration. The seed bank flora in this study was mainly composed of annuals with a few perennial ruderals. Annual species (r-selected) usually produce many seeds (Grime 2006, Moore 1980) and are often over-represented in the seed bank relative to their above-ground cover (Goodson et al. 2001). Where the vegetation growing in this type of seedbank is disturbed, germination occurs rapidly and a weed flora often results. In riverine communities after flood disturbances, annual species have an opportunity to germinate in large quantities from seeds and colonize the disturbed ground (Cho and Cho 2005). The dominant species in the above-ground plant communities (A. selengensis, M. sacchariflorus and P. japonica), predominantly K-selected, were absent from the seed bank, and these species cannot, therefore, recolonize from the soil seed bank (Moore 1980). If these species are to re-colonize after disturbance they must either do this through vegetative means (regrowth from undamaged stems, roots and rhizomes), or through transport of propagules (seeds or vegetative fragments) from outside the ecosystem via water movement, presumably within the riverine system. These dominant species, either do not produce viable seed or the seeds are very short- lived and do not produce a persistent seed bank (Moore 1980, Blom and Voesenek 1996, Bossuyt and Honnay 2008). The communities of A. selengensis, M. sacchariflorus, P. japonica, which are often destroyed by flooding, usually recover vegetatively by resprouting from buried stems, stolons or rhizomes (Cho and Cho 2005). Even small fragments of stolons and rhizomes of these species can act as propagules for asexual reproduction in these sand-bed streams (Hong et al. 2012, Deng et al. 2013).
There was very little similarity between the species composition of the soil seed bank and vegetation with Sorensen’s Similarity Indices averaging 29% (range 12 - 42%); these values are within the ranges found in other riverine systems in a range of climates (Table 4). However, there were marked variation between the different plant communities. The P. nodosa and R. palustris communities, i.e. those located at or near the sandbar had less than a quarter of the species in the standing vegetation almost certainly reflecting their more frequent and severe flood disturbance, which interferes with the formation of the persistent seed bank (Goodson et al. 2001). We can also confirm the effect of disturbance on the number of seeds in the seed bank. The number of seeds showed a tenfold variation within the seed bank with the early successional R. palustris and P. nodosa communities containing the lowest seed numbers.
Table 3. Comparison of the total number of species present in the seed banks (SB) and field vegetation survey (Veg), along with an assessment of their similarity (Sorensen’s Similarity Index) in six plant communities in the floodplain of the Cheongmicheon Stream, South Korea
The seedlings of pioneer ruderals germinate rapidly from the soil seed bank after flood disturbances (Naiman and Decamps 1997, O’Donnell et al. 2015). Once they colonize the sand bar, increased sedimentation occurs because their shoots increase flow resistance and decrease flow velocity. Within the mid- to high-elevation plant communities the flood disturbance is reduced, and perennial herbaceous species dominate (Hupp and Osterkamp 1996). Here, we confirmed the effect of this successional sequence of communities; from the pioneer communities of P. nodosa and R. palustris, at the front of sand bar to later-successional vegetation of M. sacchariflorus, and A. selengensis at the higher floodplain elevations (Cho and Cho 2005). In this study, however, no viable seeds of the late-successional species were detected in the seed bank and we have no information on how these plants regenerated under field conditions. Further research is required to investigate soil seed banks in more diverse riverine environments and at different seasons of the year, especially early spring, as well as the colonization mechanisms of the dominant species.
In conclusion, this study showed an aspect of the functional and causal links between the seed bank and the vegetation within a stream under a temperate monsoonal climate. The seed bank even though composed mainly of annual, ruderal species may be an important factor in providing rapid vegetation regeneration after flooding. The colonization by these pioneer plants can change the riparian environment so that sedimentation increases, the substrate is stabilized and improved, and colonization of later-successional, herbaceous, perennial species is facilitated (Connell and Slatyer 1977). Therefore, seed banks may be useful for the restoration of the early succession stages of riparian vegetation which is dependent on the flood disturbance (Bossuyt and Honnay 2008). However, the extant vegetation in the later-successional stages is likely to be more important than the seed bank for the maintenance of perennial, herbaceous plant species, probably via extensive root and rhizome systems.
Appendix 1. Comparison of species present in the seed banks (SB) and field vegetation survey (Veg) in six plant communities in the floodplain of the Cheongmicheon Stream, South Korea